Basics: An example workflow
Basics: An example workflow
Please make sure that you have activated the environment we created
before, and that you have an open terminal in the working directory you
have created.
A Snakemake workflow is defined by specifying rules in a Snakefile.
Rules decompose the workflow into small steps (for example, the
application of a single tool) by specifying how to create sets of
output files from sets of input files. Snakemake automatically
determines the dependencies between the rules by matching file
names.
The Snakemake language extends the Python language, adding syntactic
structures for rule definition and additional controls. All added
syntactic structures begin with a keyword followed by a code block that
is either in the same line or indented and consisting of multiple lines.
The resulting syntax resembles that of original Python constructs.
In the following, we will introduce the Snakemake syntax by creating an
example workflow. The workflow comes from the domain of genome analysis.
It maps sequencing reads to a reference genome and calls variants on the
mapped reads. The tutorial does not require you to know what this is
about. Nevertheless, we provide some background in the following
paragraph.
Background
The genome of a living organism encodes its hereditary information. It
serves as a blueprint for proteins, which form living cells, carry
information and drive chemical reactions. Differences between species,
populations or individuals can be reflected by differences in the
genome. Certain variants can cause syndromes or predisposition for
certain diseases, or cause cancerous growth in the case of tumour cells
that have accumulated changes with respect to healthy cells. This makes
the genome a major target of biological and medical research. Today, it
is often analyzed with DNA sequencing, producing gigabytes of data from
a single biological sample (for example a biopsy of some tissue). For
technical reasons, DNA sequencing cuts the DNA of a sample into millions
of small pieces, called reads. In order to recover the genome of the
sample, one has to map these reads against a known reference genome
(for example, the human one obtained during the famous human genome
project). This task
is called read mapping. Often, it is of interest where an individual
genome is different from the species-wide consensus represented with the
reference genome. Such differences are called variants. They are
responsible for harmless individual differences (like eye color), but
can also cause diseases like cancer. By investigating the differences
between the mapped reads and the reference sequence at a particular
genome position, variants can be detected. This is a statistical
challenge, because they have to be distinguished from artifacts
generated by the sequencing process.
Step 1: Mapping reads
Our first Snakemake rule maps reads of a given sample to a given reference genome.
For this, we will use the tool bwa, specifically the subcommand
bwa mem.
In the working directory, create a new file called Snakefile with an editor of your choice.
We propose to use the integrated development environment (IDE) tool Visual Studio Code, since it provides a good syntax highlighting Snakemake extension and a remote extension for directly using the IDE on a remote server.
In the Snakefile, define the following rule:rule bwa_map: input: "data/genome.fa", "data/samples/A.fastq" output: "mapped_reads/A.bam" shell: "bwa mem {input} | samtools view -Sb - > {output}"
A common error is to forget the comma between the input or output items.
Since Python concatenates subsequent strings, this can lead to
unexpected behavior.
A Snakemake rule has a name (here
bwa_map) and a number of directives,
here input, output and shell. The input and output directives
are followed by lists of files that are expected to be used or created
by the rule. In the simplest case, these are just explicit Python
strings. The shell directive is followed by a Python string containing
the shell command to execute. In the shell command string, we can refer
to elements of the rule via braces notation (similar to the Python
format function). Here, we refer to the output file by specifying
{output} and to the input files by specifying {input}. Since the
rule has multiple input files, Snakemake will concatenate them,
separated by a whitespace. In other words, Snakemake will replace
{input} with data/genome.fa data/samples/A.fastq before executing
the command. The shell command invokes bwa mem with reference genome
and reads, and pipes the output into samtools which creates a
compressed BAM
file containing the alignments. The output of samtools is redirected
into the output file defined by the rule with >.It is best practice to have subsequent steps of a workflow in separate,
unique, output folders. This keeps the working directory structured.
Further, such unique prefixes allow Snakemake to quickly discard most
rules in its search for rules that can provide the requested input. This
accelerates the resolution of the rule dependencies in a workflow.
When a workflow is executed, Snakemake tries to generate given
target files. Target files can be specified via the command line. By
executing
snakemake -np mapped_reads/A.bam
in the working directory containing the Snakefile, we tell Snakemake to
generate the target file
mapped_reads/A.bam. Since we used the -n
(or --dry-run) flag, Snakemake will only show the execution plan
instead of actually performing the steps. The -p flag instructs
Snakemake to also print the resulting shell command for illustration. To
generate the target files, Snakemake applies the rules given in the
Snakefile in a top-down way. The application of a rule to generate a
set of output files is called job. For each input file of a job,
Snakemake again (i.e. recursively) determines rules that can be applied
to generate it. This yields a directed acyclic graph
(DAG) of jobs
where the edges represent dependencies. So far, we only have a single
rule, and the DAG of jobs consists of a single node. Nevertheless, we
can execute our workflow withsnakemake --cores 1 mapped_reads/A.bam
Whenever executing a workflow, you need to specify the number of cores
to use. For this tutorial, we will use a single core for now. Later you
will see how parallelization works. Note that, after completion of above
command, Snakemake will not try to create
mapped_reads/A.bam again,
because it is already present in the file system. Snakemake only
re-runs jobs if one of the input files is newer than one of the output
files or one of the input files will be updated by another job.Step 2: Generalizing the read mapping rule
Obviously, the rule will only work for a single sample with reads in the
file
data/samples/A.fastq. However, Snakemake allows generalizing
rules by using named wildcards. Simply replace the A in the second
input file and in the output file with the wildcard {sample}, leading
torule bwa_map: input: "data/genome.fa", "data/samples/{sample}.fastq" output: "mapped_reads/{sample}.bam" shell: "bwa mem {input} | samtools view -Sb - > {output}"
Note that if a rule has multiple output files, Snakemake requires them
to all have exactly the same wildcards. Otherwise, it could happen that
two jobs running the same rule in parallel want to write to the same
file.
When Snakemake determines that this rule can be applied to generate a
target file by replacing the wildcard
{sample} in the output file with
an appropriate value, it will propagate that value to all occurrences of
{sample} in the input files and thereby determine the necessary input
for the resulting job. Note that you can have multiple wildcards in your
file paths, however, to avoid conflicts with other jobs of the same
rule, all output files of a rule have to contain exactly the same
wildcards.When executing
snakemake -np mapped_reads/B.bam
Snakemake will determine that the rule
bwa_map can be applied to
generate the target file by replacing the wildcard {sample} with the
value B. In the output of the dry-run, you will see how the wildcard
value is propagated to the input files and all filenames in the shell
command. You can also specify multiple targets, for example:snakemake -np mapped_reads/A.bam mapped_reads/B.bam
Some Bash magic
can make this particularly handy. For example, you can alternatively
compose our multiple targets in a single pass via
snakemake -np mapped_reads/{A,B}.bam
Note that this is not a special Snakemake syntax.
Bash is just
applying its brace
expansion
to the set
{A,B}, creating the given path for each element and
separating the resulting paths by a whitespace.In both cases, you will see that Snakemake only proposes to create the
output file
mapped_reads/B.bam. This is because you already executed
the workflow before (see the previous step) and no input file is newer
than the output file mapped_reads/A.bam. You can update the file
modification date of the input file data/samples/A.fastq viatouch data/samples/A.fastq
and see how Snakemake wants to re-run the job to create the file
mapped_reads/A.bam by executingsnakemake -np mapped_reads/A.bam mapped_reads/B.bam
Step 3: Sorting read alignments
For later steps, we need the read alignments in the BAM files to be
sorted. This can be achieved with the samtools
sort command. We add the following rule beneath the bwa_map rule:rule samtools_sort: input: "mapped_reads/{sample}.bam" output: "sorted_reads/{sample}.bam" shell: "samtools sort -T sorted_reads/{wildcards.sample} " "-O bam {input} > {output}"
In the shell command above we split the string into two lines, which are
however automatically concatenated into one by Python. This is a handy
pattern to avoid too long shell command lines. When using this, make
sure to have a trailing whitespace in each line but the last, in order
to avoid arguments to become not properly separated.
This rule will take the input file from the
mapped_reads directory and
store a sorted version in the sorted_reads directory. Note that
Snakemake automatically creates missing directories before jobs are
executed. For sorting, samtools requires a prefix specified with the
flag -T. Here, we need the value of the wildcard sample. Snakemake
allows to access wildcards in the shell command via the wildcards
object that has an attribute with the value for each wildcard.When issuing
snakemake -np sorted_reads/B.bam
you will see how Snakemake wants to run first the rule
bwa_map and
then the rule samtools_sort to create the desired target file: as
mentioned before, the dependencies are resolved automatically by
matching file names.Step 4: Indexing read alignments and visualizing the DAG of jobs
Snakemake uses the Python format mini
language
to format shell commands. Sometimes you have to use braces (
{}) for
something else in a shell command. In that case, you have to escape them
by doubling, for example when relying on the bash brace expansion we
mentioned above: ls {{A,B}}.txt.Next, we need to use samtools again to index
the sorted read alignments so that we can quickly access reads by the
genomic location they were mapped to. This can be done with the
following rule:
rule samtools_index: input: "sorted_reads/{sample}.bam" output: "sorted_reads/{sample}.bam.bai" shell: "samtools index {input}"
Having three steps already, it is a good time to take a closer look at
the resulting directed acyclic graph (DAG) of jobs. By executing
snakemake --dag dot sorted_reads/{A,B}.bam.bai | dot -Tsvg > dag.svg
If you are running this tutorial on GitPod, you can easily view the resulting
dag.svg by right-clicking on the file in the explorer panel on the left and
selecting Open With -> Preview.we create a visualization of the DAG using the
dot command
provided by Graphviz. For the given target
files, Snakemake specifies the DAG in the dot language and pipes it into
the dot command, which renders the definition into SVG
format. The
rendered DAG is piped into the file dag.svg and will look similar to
this: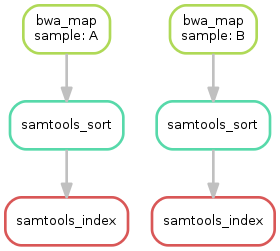
The DAG contains a node for each job with the edges connecting them
representing the dependencies. The frames of jobs that don't need to be
run (because their output is up-to-date) are dashed. For rules with
wildcards, the value of the wildcard for the particular job is displayed
in the job node.
Exercise
Run parts of the workflow using different targets. Recreate the DAG
and see how different rules' frames become dashed because their
output is present and up-to-date.
Step 5: Calling genomic variants
The next step in our workflow will aggregate the mapped reads from all
samples and jointly call genomic variants on them. For the variant
calling, we will combine the two utilities
samtools and
bcftools. Snakemake provides a helper
function for collecting input files that helps us to describe the
aggregation in this step. With
expand("sorted_reads/{sample}.bam", sample=SAMPLES)
we obtain a list of files where the given pattern
"sorted_reads/{sample}.bam" was formatted with the values in a given
list of samples SAMPLES, i.e.["sorted_reads/A.bam", "sorted_reads/B.bam"]
The function is particularly useful when the pattern contains multiple
wildcards. For example,
expand("sorted_reads/{sample}.{replicate}.bam", sample=SAMPLES, replicate=[0, 1])
would create the product of all elements of
SAMPLES and the list
[0, 1], yielding["sorted_reads/A.0.bam", "sorted_reads/A.1.bam", "sorted_reads/B.0.bam", "sorted_reads/B.1.bam"]
Here, we use only the simple case of
expand. We first let Snakemake
know which samples we want to consider. Remember that Snakemake works
backwards from requested output, and not from available input. Thus, it
does not automatically infer all possible output from, for example, the
fastq files in the data folder. Also remember that Snakefiles are in
principle Python code enhanced by some declarative statements to define
workflows. Hence, we can define the list of samples ad-hoc in plain
Python at the top of the Snakefile:SAMPLES = ["A", "B"]
If you name input or output files like above, their order won't be
preserved when referring to them as
{input}. Further, note that named
and unnamed (i.e., positional) input and output files can be combined,
but the positional ones must come first, equivalent to Python functions
with keyword arguments.Later, we will learn about more sophisticated ways like config
files. But for now, this is enough so that we can add the following
rule to our Snakefile:
rule bcftools_call: input: fa="data/genome.fa", bam=expand("sorted_reads/{sample}.bam", sample=SAMPLES), bai=expand("sorted_reads/{sample}.bam.bai", sample=SAMPLES) output: "calls/all.vcf" shell: "bcftools mpileup -f {input.fa} {input.bam} | " "bcftools call -mv - > {output}"
With multiple input or output files, it is sometimes handy to refer to
them separately in the shell command. This can be done by specifying
names for input or output files, for example with
fa=.... The files
can then be referred to in the shell command by name, for example with
{input.fa}. For long shell commands like this one, it is advisable
to split the string over multiple indented lines. Python will
automatically merge it into one. Further, you will notice that the
input or output file lists can contain arbitrary Python statements,
as long as it returns a string, or a list of strings. Here, we invoke
our expand function to aggregate over the aligned reads of all
samples.Exercise
obtain the updated DAG of jobs for the target file
calls/all.vcf,
it should look like this: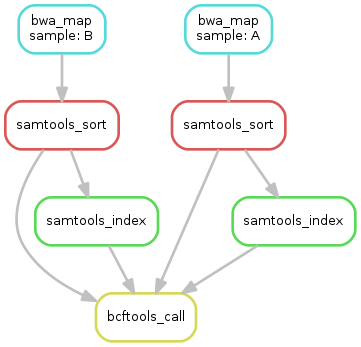
Step 6: Using custom scripts
Usually, a workflow not only consists of invoking various tools, but
also contains custom code to for example calculating summary statistics or
create plots. While Snakemake also allows you to directly
write Python code inside a rule, it is usually reasonable to move such logic
into separate scripts. For this purpose, Snakemake offers the
script directive.
Add the following rule to your Snakefile:rule plot_quals: input: "calls/all.vcf" output: "plots/quals.svg" script: "scripts/plot-quals.py"
snakemake.input and snakemake.output always contain a list of file
names, even if the lists each contain only one file name. Therefore, to
refer to a particular file name, you have to index into that list.
snakemake.output[0] will give you the first element of the output file
name list, something that always has to be there.With this rule, we will eventually generate a histogram of the quality
scores that have been assigned to the variant calls in the file
calls/all.vcf. The actual Python code to generate the plot is hidden
in the script scripts/plot-quals.py. Script paths are always relative
to the referring Snakefile. In the script, all properties of the rule
like input, output, wildcards, etc. are available as attributes of
a global snakemake object. Create the file scripts/plot-quals.py,
with the following content:import matplotlib matplotlib.use("Agg") import matplotlib.pyplot as plt from pysam import VariantFile quals = [record.qual for record in VariantFile(snakemake.input[0])] plt.hist(quals) plt.savefig(snakemake.output[0])
It is best practice to use the script directive whenever an inline code
block would have more than a few lines of code.
Although there are other strategies to invoke separate scripts from your
workflow (for example, invoking them via shell commands), the benefit of
this is obvious: the script logic is separated from the workflow logic
(and can even be shared between workflows), but boilerplate code like
the parsing of command line arguments is unnecessary.
Apart from Python scripts, it is also possible to use R scripts. In R
scripts, an S4 object named
snakemake analogous to the Python case
above is available and allows access to input and output files and other
parameters. Here, the syntax follows that of S4 classes with attributes
that are R lists, for example we can access the first input file with
snakemake@input[[1]] (note that the first file does not have index 0
here, because R starts counting from 1). Named input and output files
can be accessed in the same way, by just providing the name instead of
an index, for example snakemake@input[["myfile"]].For details and examples, see the
snakefiles-external_scripts section in
the Documentation.Step 7: Adding a target rule
So far, we always executed the workflow by specifying a target file at
the command line. Apart from filenames, Snakemake also accepts rule
names as targets if the requested rule does not have wildcards. Hence,
it is possible to write target rules collecting particular subsets of
the desired results or all results. Moreover, if no target is given at
the command line, Snakemake will define the first rule of the
Snakefile as the target. Hence, it is best practice to have a rule
all
at the top of the workflow which has all typically desired target files
as input files.Here, this means that we add a rule
rule all: input: "plots/quals.svg"
to the top of our workflow. When executing Snakemake with
snakemake -n
In case you have mutliple reasonable sets of target files, you can add
multiple target rules at the top of the Snakefile. While Snakemake will
execute the first per default, you can target any of them via the
command line (for example,
snakemake -n mytarget).the execution plan for creating the file
plots/quals.svg, which
contains and summarizes all our results, will be shown. Note that, apart
from Snakemake considering the first rule of the workflow as the default
target, the order of rules in the Snakefile is arbitrary and does not
influence the DAG of jobs.Exercise
- Create the DAG of jobs for the complete workflow.
- Execute the complete workflow and have a look at the resulting
plots/quals.svg. - Snakemake provides handy flags for forcing re-execution of parts of the workflow. Have a look at the command line help with
snakemake --helpand search for the flag--forcerun. Then, use this flag to re-execute the rulesamtools_sortand see what happens. - Snakemake displays the reason for each job (under
reason:). Perform a dry-run that forces some rules to be reexecuted (using the--forcerunflag in combination with some rulename) to understand the decisions of Snakemake.
After having a look at the summary, please go on with the advanced part of the tutorial.
Summary
In total, the resulting workflow looks like this:
SAMPLES = ["A", "B"] rule all: input: "plots/quals.svg" rule bwa_map: input: "data/genome.fa", "data/samples/{sample}.fastq" output: "mapped_reads/{sample}.bam" shell: "bwa mem {input} | samtools view -Sb - > {output}" rule samtools_sort: input: "mapped_reads/{sample}.bam" output: "sorted_reads/{sample}.bam" shell: "samtools sort -T sorted_reads/{wildcards.sample} " "-O bam {input} > {output}" rule samtools_index: input: "sorted_reads/{sample}.bam" output: "sorted_reads/{sample}.bam.bai" shell: "samtools index {input}" rule bcftools_call: input: fa="data/genome.fa", bam=expand("sorted_reads/{sample}.bam", sample=SAMPLES), bai=expand("sorted_reads/{sample}.bam.bai", sample=SAMPLES) output: "calls/all.vcf" shell: "bcftools mpileup -f {input.fa} {input.bam} | " "bcftools call -mv - > {output}" rule plot_quals: input: "calls/all.vcf" output: "plots/quals.svg" script: "scripts/plot-quals.py"
Now, please go on with the advanced part of the tutorial.